Abstract
Amyotrophic lateral sclerosis (ALS), an adult onset, fatal neurodegenerative disease, has as a cardinal pathogenic feature the selective death of motor neurons (MNs) at the cortex, brainstem, and spinal cord. In this review we focus on four aspects: (i) the hypothesis of disease propagation through the cerebrospinal fluid (CSF); (ii) the distortion of the exocytotic release of neurotransmitters at the sympathoadrenal axis; (iii) the ultrastructural and functional alterations of mitochondria from adrenal medullary chromaffin cells (CCs); and (iv) the purinergic P2X7 receptor (P2X7R) as a potential target for neuroprotection.
Concerning disease propagation from one to another area of the central nervous system (CNS), the pattern of clinical progression suggests that the disease spreads centrifugally. This indicates that a kind of toxin agent may be released and propagated through the CSF. In our laboratory we found that CSF from ALS patients exerted toxic effects on cultured cortical MNs.
In CCs, we found deep distortions of the exocytotic kinetics and the exocytotic fusion pore in the process of catecholamine release, in the SOD1G93A mouse model of ALS. Furthermore, we found that these alterations could be related to the accumulation of mutated SOD1 into mitochondria; this resulted in mitochondrial depolarization, excess production of reactive oxygen species and deficiency in oxidative phosphorylation.
Finally, we discuss recent data on the potential therapeutic effect of compound JNJ-47965567, a blocker of P2X7Rs known to be central-stage in neuroinflammation. Upon its chronic administration to SOD1G93A, we found that the compound delayed disease onset but only in females mice.
In conclusion, why MN selectively die in ALS disease, remains a mystery; On the other hand, it seems that other cell types are also affected, particularly at the sympathoadrenal axis. As disease pathogenesis remains obscure, the search of therapeutic targets to slow disease progression in ALS, remains puzzling.Resumen
La esclerosis lateral amiotrófica (ELA) es una enfermedad de la edad adulta. Su patogénesis se caracteriza principalmente por la muerte selectiva de las motoneuronas (MN) en corteza, tronco del encéfalo y médula espinal. En esta revisión nos centramos en los siguientes aspectos: (i) la hipótesis de la propagación de la enfermedad vía líquido cefalorraquídeo (LCR); (ii) la distorsión de la liberación de neurotransmisores a nivel del eje simpatoadrenal; (iii) las alteraciones de las mitocondrias en las células cromafines (CCs) de la médula suprarrenal; (iv) el receptor purinérgico P2X7 (P2X7R) como potencial nueva diana para la neuroprotección.
Con respecto a la propagación de la ELA a otras áreas del sistema nervioso central (SNC), el patrón de progresión clínica indica que la enfermedad se extiende centrífugamente. Ello sugiere que una toxina del área inicial afectada podría ser vehiculada a otros lugares del SNC. De hecho, en nuestro laboratorio encontramos que el LCR de pacientes con ELA (LCR/ELA) ejercía efectos tóxicos en cultivos de MN.
En las CCs encontramos alteraciones drásticas en la cinética del poro de fusión durante la exocitosis, en el ratón SOD1G93A modelo de ELA. Estas alteraciones podrían deberse a la acumulación mitocondrial de la SOD1 mutada, con despolarización, aumento de la producción de especies reactivas de oxígeno y deficiencia en la fosforilación oxidativa.
Finalmente, analizamos algunos datos sobre el potencial terapéutico del compuesto JNJ-47965567, un bloqueante del P2X7R. La administración crónica del compuesto al ratón SOD1G93A retrasó el inicio de la enfermedad en las hembras.
En conclusión, la causa de la muerte selectiva de la MN en la ELA continúa siendo desconocida, por lo que resulta difícil identificar nuevas dianas terapéuticas para desarrollar fármacos que enlentezcan su progresión. Por otra parte, parece que la patología se extiende a otras células, particularmente las del eje simpatoadrenal.Keywords: Amyotrophic lateral sclerosis; Sympathoadrenal axis; Exocytosis; Cerebrospinal fluid toxicity; Mitochondria disruption; Oxidative stress; P2X7 receptors; neuroinflammation.
Palabras clave: Esclerosis lateral Amiotrófica; Eje simpatoadrenal; Exocitosis; Toxicidad en el líquido cefalorraquídeo; Alteración de la mitocondria; Receptor P2X7; Neuroinflamación.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS), an adult onset and fatal neurodegenerative disease, has as a cardinal pathogenic feature the selective death of motor neurons (MNs) at the cortex, brainstem, and spinal cord. Hence, ALS characteristically presents with muscle weakness, atrophy, and spasticity, leading to paralysis, respiratory insufficiency, and death within 3-5 years of diagnosis. For 25 years, the only available treatment has been riluzole, that interferes with glutamatergic neurotransmission; however, clinical trials (CTs) demonstrated a modest 2-3 months extension of patient survival (1). Recently, the antioxidant edaravone has been approved in Japan and USA, but not in Europe (2).
ALS actual prevalence is 4-6/105, 10% of patients having a positive family history (fALS) (3); 25% of fALS patients have mutations in the gene for Cu2+/Zn2+ superoxide dismutase 1 (SOD1) (4). Nearly 200 mutations of the SOD1 gene have been found and, curiously, they do not impair the enzyme activity of SOD1; rather, they elicit its pathological aggregation in the cytosol or mitochondria, leading to MN toxicity (5). Around 40 mutations have also been identified in the TARDBP gene which encodes for the transactive response (TAR)-DNA binding protein 43 (TDP-43) (6). Of interest is the observation that 97% of ALS patients present TDP-43 inclusions. Other relevant mutations have been found in the gene Fused in Sarcoma/ Translocated in Sarcoma (FUS) (7) and the gene hexanucleotide repeats (GGGGCC) in intron1 of chromosome 9 open reading frame 72 (C9orf72) (8).
Those ALS mutations are leading causes of disease and based on them, various mouse models have been developed. The first model that was generated, and the most frequently used is the SOD1G93A mouse (substitution of the glycine at position 93 by alanine) in SOD1 that develop a progressive MN disease resembling the disease progression in humans, with adult onset and reduced lifespan (4). Pathogenically, these mice show early onset microgliosis and astrogliosis, glutamate-elicited excitotoxicity, deficit in axonal transport, axonal denervation, protein aggregation, aberrant neurofilament processing, and mitochondrial vacuolization. All these processes lead to selective MN loss in the spinal cord, marked wasting, paralysis, and atrophy of the forelimbs and hindlimbs. Thus, this SOD1G93A mouse model exhibits almost all the hallmarks of ALS (9).
In this review we focus on four aspects of the pathogenesis and drug therapy of ALS namely: (i) the hypothesis of disease propagation through the cerebrospinal fluid (CSF); (ii) the distortion of the exocytotic release of neurotransmitters at the sympathoadrenal axis; (iii) the ultrastrural and functional alterations of mitochondria from adrenal medullary chromaffin cells (CCs); and (iv) the purinergic P2X7 receptor (P2X7R) as a novel potential drug target for neuroprotection.
Hypothesis on the ALS propagation through the cerebrospinal fluid
This hypothesis deals with the enigma on why MNs become selectively vulnerable and die, giving rise to the progressive evolution of disease pathogenesis. The pattern of clinical progression suggests that the disease spreads centrifugally from an affected region to an adjacent area; this indicates that a kind of toxic agent generated in the affected area may be released into the extracellular space and the CSF to cause the injury of adjacent MNs. Thus, some authors found that in vitro incubation of neurons or neuronal cell lines with CSF from ALS patients (CSF/ALS) caused cell damage (10), but others did not find such neurotoxic effects (11).
In our laboratory we tested the effects of CSF from 14 controls (CSF/control) and 29 ALS patients (CSF/ALS) on primary cultures of rat brain cortical MNs. CSF/ALS caused a significant 20% loss of MNs. A reasonable conjecture was that these neurotoxic effects could be due to calcium-dependent excitotoxicity elicited by excess glutamate present in CSF/ALS (12). However, we did not find this to be the case as the neurotoxic effects of CSF/ALS were not reversed by the glutamate NMDA receptor blocker MK801 (13).
In subsequent experiments, we found that resveratrol, memantine, minocycline and lithium exerted protection against the MN lesion elicited by CSF/ALS. And surprisingly, we also found that riluzole did not afford such protection; in fact, this drug antagonized the neuroprotectant effects of the other compounds (14). This suggested that routine treatment with riluzole of ALS patients enrolled in clinical trials could be a confounding factor in the neurological outcomes attributed to the novel compound being tested.
We also attempted to correlate the MN damage elicited by individual CSFs with survival of patients. However, no differences between patients with and without CSF-elicited cytotoxicity exhibited a positive correlation, regarding mean time from symptom onset to diagnosis or to death, as well as time to respiratory assistance or to gastrostomy. However, Cox regression analysis, bulbar onset, gender, or age at onset (but not cytotoxicity) were associated with a lower risk of survival (15).
Finally, we analysed the effects of intracerebroventricular (ICV) infusion with osmotic minipumps, of cytotoxic CSF/ALS in rats. This led to brain tissue damage similar to that found in sporadic ALS (16).
In summary, the studies here commented carried out in the laboratories of Jorge Matías-Guiu and Antonio G. García suggest that ALS pathology could be propagated from its onset at MN nuclei in the cortex, brainstem, or spinal cord, to other areas through as yet unidentified toxic agent that propagates through the CSF.
Beyond motor neuron damage in ALS pathogenesis
Traditionally, ALS has been considered as an exclusive MN pathology. However, the concept of “non-autonomous motor neuron death” is emerging to imply other neurons and cell types in ALS pathology (17). In fact, various proteins that are altered in ALS undergo ubiquitous alterations. For instance, in the SOD1G93A mouse model of fALS, the expression of mutated SOD1G93A occurs in liver and skeletal muscle (18). Curiously, the disease did not develop when the mutant SOD1 was exclusively expressed in either MNs (19) or astrocytes (20). Thus, ALS is being considered as a multisystemic disease with metabolic and physiological changes occurring in different peripheral cell types and tissues; they may act mutually and synergistically to provoke ALS onset and to define its time course and severity (21). Therefore, the hypothesis concerning muscle denervation before MN degeneration suggests an initial lesion of the distal axon that progresses in a retrograde “dying back” pattern (22); this opposes the classical view of MN “dying forward” (23). In this context, it is relevant that microRNAs released from peripheral tissues may induce damage at muscle endplates and the spinal cord (24).
The sympathoadrenal axis in ALS and other neurodegenerative diseases
In a review on the alterations undergone by the exocytotic release of catecholamines in adrenal medullary CCs of transgenic mouse models of neurodegenerative diseases (25) we rose the following hypothesis: the peripheral adrenal medulla senses some of the pathogenic changes undergone by central neurons during the course of neurodegenerative diseases. Those changes may occur in one or more of the following pathways: (i) propagation via the cortico-hypothalamic-sympathoadrenal axis of some of the pathogenic features related to synaptopathies and altered exocytosis occurring centrally; (ii) distorted regulation at central command neurons that modulate the firing rate of the sympathoadrenal axis; (iii) mutant proteins specific of each neurodegenerative disease that are expressed in CCs and display some functional role in CCs themselves; and (iv) CCs are extremely sensitive to changes in blood pH, hormones and various metabolites and so, the alterations of some of these factors in neurodegenerative diseases may alter CC function. These pathogenic routes will at last lead to altered exocytosis; in so doing, patients suffering a neurodegenerative disease will poorly adapt to stressful conflicts inherent to impaired functional autonomic performance (Fig. 1).
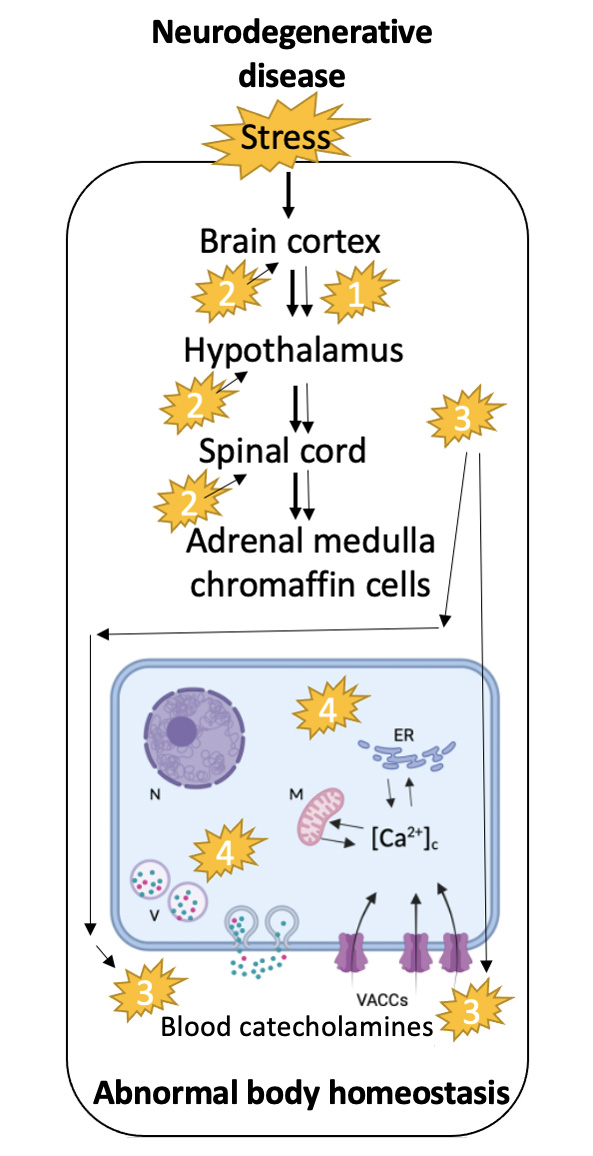
This is particularly relevant as ALS patients undergoing sudden death when they are unable to compensate for cardiorespiratory arrest because the failure of the autonomic nervous system. This is especially relevant since ALS patients suffer sudden death when they are unable to compensate for cardiorespiratory arrest due to autonomic nervous system failure.
In line with this hypothesis are some observations emanating from studies done in ALS patients or in transgenic mouse models of ALS. For example, cardiac sympathetic denervation and alterations at the muscle endplate have been found at early disease stages (26). Subsequent articles also reported early impairment of the autonomic nervous system in ALS (27); such alterations were revealed as subclinical early dysautonomic events at cardiovascular, salivary, skin, lachrymal and gastrointestinal functions. These alterations have also been disclosed in SOD1G93A mice (28). In line with these observations is the augmented noradrenaline levels in blood and CSF of ALS patients (29) and in ALS mouse models (30). This is also in line with the presence of sympathetic hyperactivity in ALS patients (26); however, in another report, no change in sympathetic activity was reported (31). Later studies found that ALS patients were hypermetabolic, involving mitochondrial energy production and sympathoadrenal activation (32). Finally, a study in the SOD1G93A mouse model of ALS found progressive alterations in the axonal transport of neurosecretory products from neuronal perikarya to nerve terminals (33).
These various disturbances in neurosecretory systems could have in common a synaptopathy that drives a disturbed exocytotic machinery and the last steps of exocytosis. To explore these questions the sympathetic neuronal-like CC has been used during the last decade or so, to inquire about the alterations of ion channels, cell excitability and exocytosis in transgenic mouse models of neurodegenerative diseases (25). Thus, altered exocytosis either in its kinetics or in the fusion pore formation and expansion, has been investigated in CCs of the APP/PS1 mouse model of Alzheimer’s disease (AD) (34), in the knockout mouse model of huntingtin-associated protein 1 (HAP1), a model of Huntington’s disease (HD) (35), in mice carrying specific mutations in proteins of the exocytotic machinery, in the SOD1G93A mouse model of ALS (36), and in the R6/1 mouse model of HD (37).
Disturbed exocytosis in chromaffin cells from the SOD1G93A mouse model of ALS
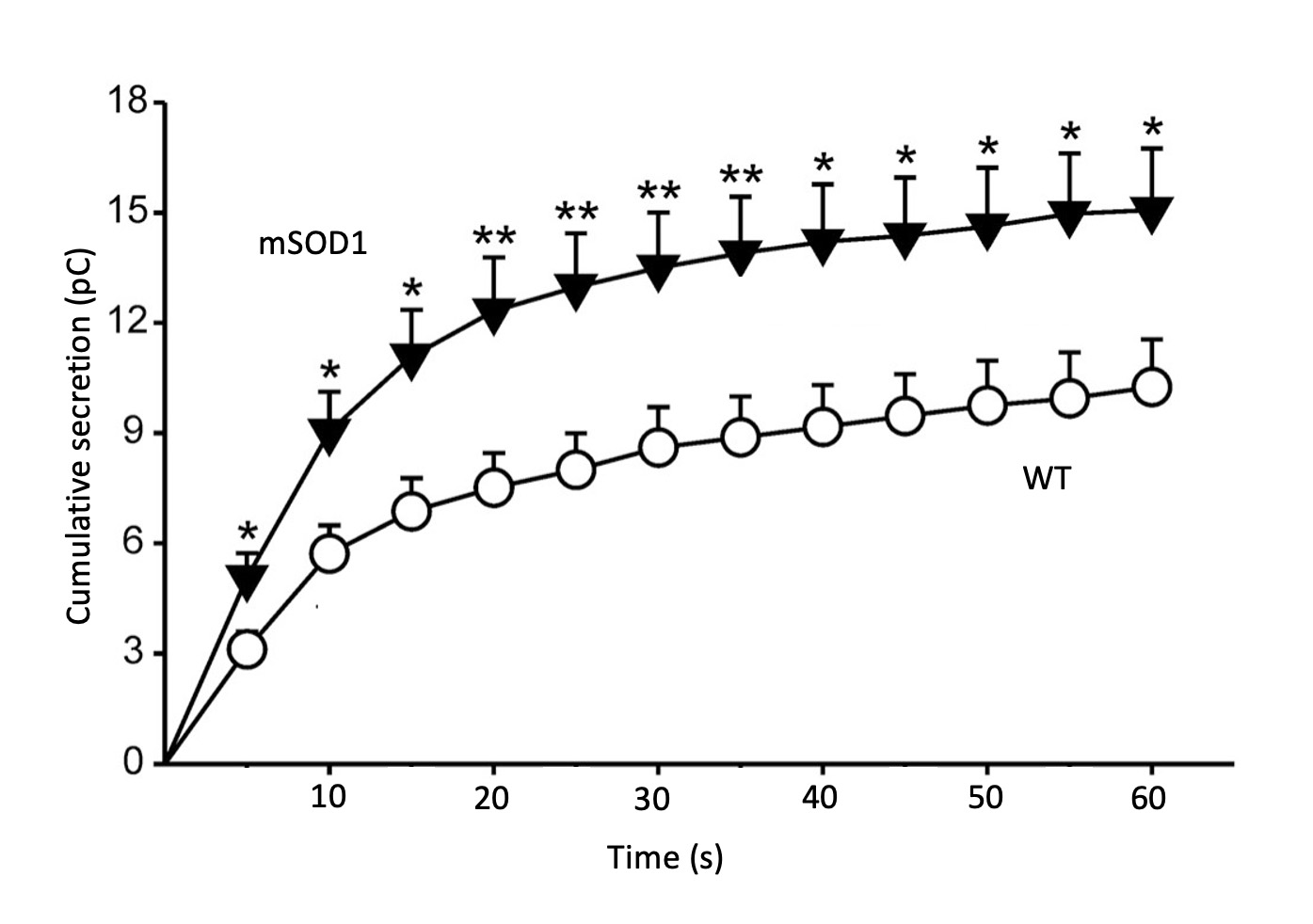
In a thorough study done in CCs of SOD1G93A mice, we investigated various ion currents and cell excitability using the patch-clamp technique (38), as well as the quantal release of catecholamines at the single-cell level using the online amperometric recording of secretory spike events with a carbon fibre microelectrode (39). Upon stimulation with acetylcholine (ACh) an initial spike burst of secretion occurred in wildtype (WT) as well as in SOD1G93A cells; this was followed by a period of slower secretion rates in both cell types. Although the number of spikes was similar, the cumulative secretion was however substantially higher in SOD1G93A cells. Also, the tau of activation of the exocytotic rate was faster in the SOD1G93A CCs (Fig. 2). The kinetic analysis of single spikes (equivalent to the quantal release of single catecholamine-storing vesicles) indicated differences in SOD1G93A CCs versus WT CCs, namely: 35.9 lower rise rate, 60.7 longer decay time, 55.3 longer half-width, 16.6 lower amplitude, and 52.2 % higher quantal size. This indicated a slower but higher exocytotic release of catecholamines per single vesicle in SOD1G93A cells, with respect to WT cells.
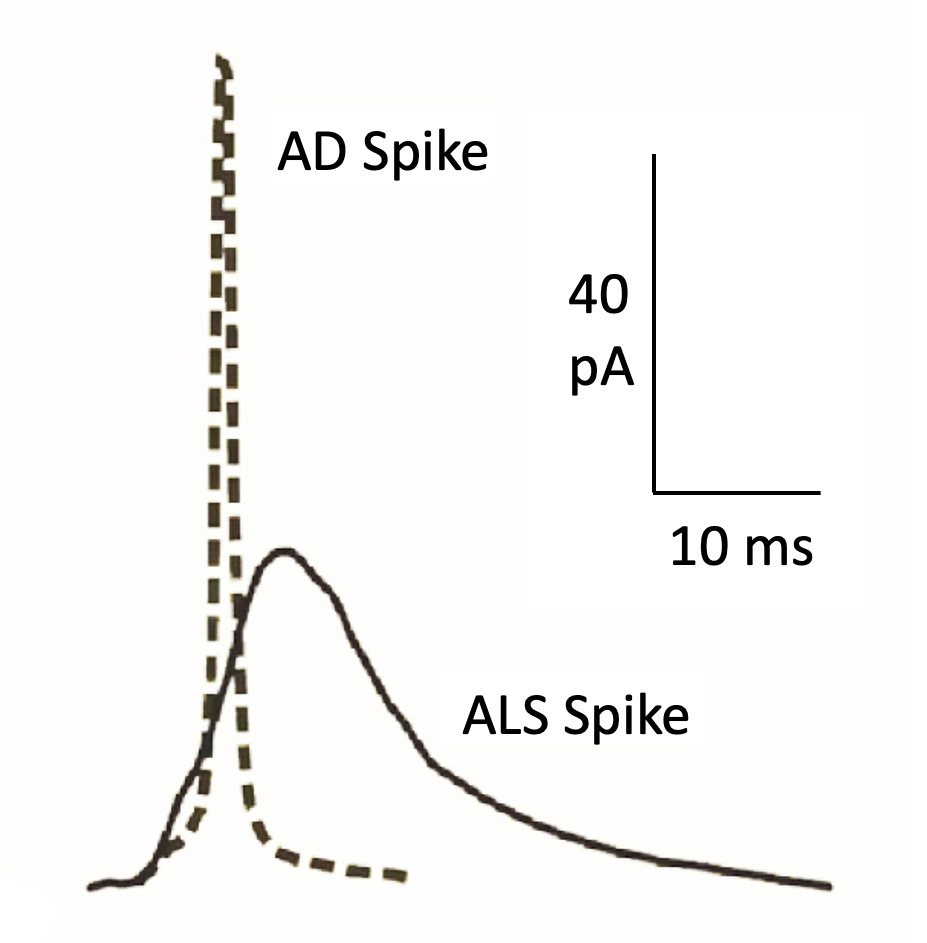
Of interest was the comparison of the kinetics of single exocytotic spikes obtained from CCs of the APP/PS1 mouse model of AD and the SOD1G93A mouse model of ALS. The averaged AD spike has higher Imax and faster decay time with decreased quantal size, suggesting a lower catecholamine content per vesicle, compared with WT cells. On the other hand, the averaged amperometric spikes of SOD1G93A CCs has lower amplitude and slower activation and decay rates, indicating a slower but higher total secretion. The drastic differences between AD and SOD1G93A spikes can be observed in Fig. 3; much faster and higher amplitude of the AD spike, with respect the SOD1G93A spike. This suggests that the different kinetics of exocytosis could potentially serve as a biomarker of AD and ALS, respectively.
Altered structure and function of mitochondria in chromaffin cells of SOD1G93A mouse model
Alterations of mitochondria structure and bioenergetic functions are considered to be central stage in the pathogenesis of neurodegenerative diseases. ALS patient’s postmortem studies revealed structural neurofibrillary alterations with the presence of vacuoles in axons and dendrites; these pathological features were associated to damaged mitochondria (40). Similar alterations were observed in mouse models carrying SOD1 mutations. Mitochondria are the source of cellular energy; they are also implicated in the intracellular calcium homeostasis, calcium-mediated excitotoxicity, and in the intrinsic apoptotic pathway (41). All these functions are affected in ALS and thus, mitochondria are being considered central stage in disease pathogenesis; in fact, ALS is currently considered as a secondary mitochondriopathy (42).
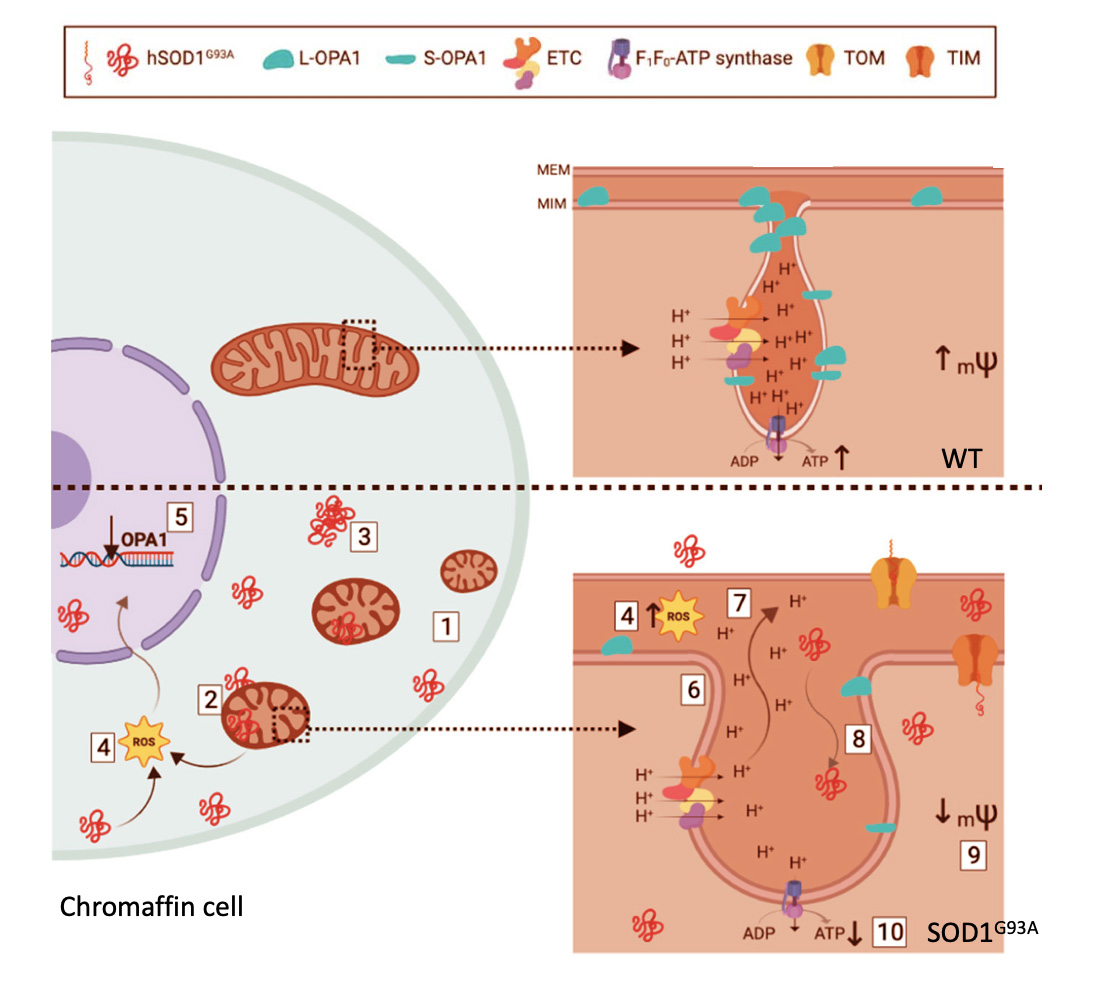
In the adrenal CC, mitochondria play a crucial role in the dissipation of the subplasmalemmal high calcium microdomains (HCMDs) that build up during cell stimulation; these are required to trigger fast exocytosis (43). Hence, if mitochondria are depolarized, the Ca2+ uptake into their matrix is blocked; in this manner, the HCMD is not cleared up and the exocytotic response is potentiated (43). In the light of these relevant functions of mitochondria in CCs and the alterations undergone by brain mitochondria in ALS, we felt of interest to perform an extensive ultrastructural and functional analysis of mitochondria in CCs of the SOD1G93A mouse model of ALS, at presymptomatic and symptomatic stages (44). In this study we found the following changes in CCs from SOD1G93A mice with respect to WT mice (Fig. 4) (i) higher number of mitochondria with smaller size; (ii) accumulation of mutated SOD1 in mitochondria, cytoplasm and nuclei; (iii) formation of mutated SOD1 aggregates; (iv) increased production of free radicals; (v) decreased expression of the gene coding for OPA1; (vi) augmented size of mitochondrial cristae junctions; (vii) diffusion of cristae components and ions towards the intermembrane space; (viii) diffusion of big molecules and proteins such as hSOD1G93A inside cristae; (ix) loss of mitochondrial membrane potential (mψ) with deficiency in oxidative phosphorylation (OXPHOS). Of interest is the observation that several of these changes were present at pre-disease 2-month-old SOD1G93A mice; as phenotypic symptoms appeared (at 3-months-old onwards), the pathological features accumulated and were more visible.
Purinergic P2X7 receptors: a potential new druggable target in ALS
From a pathogenic perspective, ALS is being considered as a multifactorial and multisystem disorder. Multiple signaling pathways have been implicated in MN death. Thus, calcium dyshomeostasis and glutamate-dependent excitotoxity, altered mitochondrial calcium handling and augmented oxidative stress have been implicated in disease pathogenesis. More recently, purinergic receptors P2X7 (P2X7Rs) are being explored as a druggable target linked to the neuroinflammatory state underlying ALS pathogenesis (45).
The P2X7R is a nonselective ligand-gated ion channel permeable to Na+, Ca2+ and K+; this receptor plays a key role in neuroinflammation. Its activation by the high concentrations of ATP released by lesioned neurons results in the assembly of the NLRP3 inflammasome complex in microglia and the subsequent release of pro-inflammatory cytokines such as IL-1β (46).
The following observations support the view that P2X7Rs play a major role in ALS pathogenesis: (i) upregulation of P2X7Rs with disease progression in patients; (ii) in vitro challenging with ATP triggers an astrocyte neurotoxic behavior, resulting in MN death that is prevented by P2X7R blocker brilliant blue G (BBG) (47); (iii) stimulation of P2X7Rs from microglia of SOD1G93A mice lead to stimulation of NADPH oxidase 2 (NOX2) and kinase ERK1/2, with ROS overproduction (48); (iv) short stimulation of P2X7Rs activated autophagy and increased anti-inflammatory markers in microglia of SOD1G93A mice, the so-called M2 microglia; in contrast, prolonged stimulation impairs autophagy, indicating a change to inflammatory M1 microglia (49). All these findings have generated the hypothesis that the blockade of P2X7Rs could mitigate neuroinflammation and hence, slow down ALS progression. Recently, we tested this hypothesis with the chronic treatment with the potent P2X7R blocker JNJ-47965567, of SOD1G93A mice. This treatment delayed disease onset, reduced body weight loss, improved motor coordination and phenotypic score only in female mice; treatment did not affect lifespan (50).
From a pathogenic perspective, ALS is being
From the literature and the experiments from our laboratories here reported it seems that the question on why MNs selectively die in ALS, remains unanswered. On the other hand, disease propagation from a CNS area through the CSF is a challenging hypothesis but with few data to support it. However, in spite that ALS is being considered as a MN disease, other cell types are also damaged. This is the case of CCs of the sympathoadrenal axis that in the SOD1G93A mouse model of ALS, undergo several alterations in the kinetics of the exocytotic release of catecholamines that could be linked to distorted ultrastructural and functional changes of CC mitochondria; of relevance was the observation that several of the changes in mitochondrial structure and function, as well as on exocytosis, were already present at pre-disease stages. During the last few years, the P2X7R blockade as a strategy to slow disease progression, has been explored in various laboratories, including ours; we found that the blocker of P2X7Rs JNJ-47965567, chronically administered to SOD1G93A mice, delayed disease onset, although this was only observed in females.
From a perspective point of view, it should be emphasized that unless disease pathogenesis is clarified, therapeutic targets to combat efficiently the fatal ALS will not be identified. One interesting approach relates to the fact several cell signaling pathways (calcium dyshomeostasis, mitochondrial damage, catecholamine release) seem to be linked to disease pathogenesis. Thus, we propose that drugs acting on three of those pathogenic pathways, if given a in combination to SOD1G93A mice and/or other mouse models of ALS, should be more effective in delaying disease onset and/or in slowing down disease progression once the symptoms have commenced. Some of those pathways to be targeted with drugs could rely on glutamate excitotoxicity, oxidative stress, mitochondrial bioenergetics, exocytosis and neuroinflammation, among several others.
BIBLIOGRAPHY
- Rowland LP. Riluzole for the treatment of amyotrophic lateral sclerosis-too soon to tell? N Engl J Med. 1994; 330(9): 636-637.
- Cruz MP. Edaravone (Radicava): a novel neuroprotective agent for the treatment of amyotrophic lateral sclerosis. P T. 2018; 43(1): 25-28.
- Mulder DW, Kurland LT, Offord KP, Beard CM. Familial adult motor neuron disease: amyotrophic lateral sclerosis. Neurology. 1986; 36(4): 511-517.
- Rosen DR, Siddique T, Patterson D et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993; 362(6415): 59-62.
- Monk PN, Shaw PJ. ALS: life and death in a bad neighborhood. Nat Med. 2006; 12(8): 885-887.
- Arai T, Hasegawa M, Akiyama H et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis.
- Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009; 136(6): 1001-1004.
- DeJesus-Hernández M, Mackenzie IR, Boeve BF et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011; 72(2): 245-256.
- Lutz C. Mouse models of ALS: Past, present and future. Brain Res. 2018; 1693(Pt A): 1- 10. 13. Terro F, Lesort M, Viader F, Ludolph A, Hugon J. Antioxidant drugs block in vitro the neurotoxicity of CSF from patients with amyotrophic lateral sclerosis. Neuroreport. 1996; 7(12): 1970-1972.
- Iwasaki Y, Ikeda K, Shiojima T, Tagaya M, Kinoshita M. Amyotrophic lateral sclerosis cerebrospinal fluid is not toxic to cultured spinal motor neurons. Neurol Res. 1995; 17(5): 393-395.
- Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009; 11(7): 1587-1602.
- Yánez M, Galán L, Matías-Guiu J et al. CSF from amyotrophic lateral sclerosis patients produces glutamate independent death of rat motor brain cortical neurons: protection by resveratrol but not riluzole. Brain Res. 2011; 1423: 77-86.
- Yánez M, Matías-Guiu J, Arranz-Tagarro JA et al. The neuroprotection exerted by memantine, minocycline and lithium, against neurotoxicity of CSF from patients with amyotrophic lateral sclerosis, is antagonized by riluzole. Neurodegener Dis. 2014; 13(2-3): 171-179.
- Galán L, Matías-Guiu J, Matías-Guiu JA et al. Cerebrospinal fluid cytotoxicity does not affect survival in amyotrophic lateral sclerosis. Acta Neurol Scand. 2017; 136(3): 212-216.
- Gómez-Pinedo U, Galán L, Yánez M et al. Histological changes in the rat brain and spinal cord following prolonged intracerebroventricular infusion of cerebrospinal fluid from amyotrophic lateral sclerosis patients are similar to those caused by the disease. Neurologia (Engl Ed). 2018; 33(4): 211-223.
- Ruffoli R, Biagioni F, Busceti CL et al. Neurons other than motor neurons in motor neuron disease. Histol Histopathol. 2017; 32(11): 1115-1123.
- Pansarasa O, Rossi D, Berardinelli A, Cereda C. Amyotrophic lateral sclerosis and skeletal muscle: an update. Mol Neurobiol. 2014; 49(2): 984-990.
- Lino MM, Schneider C, Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 2002; 22(12): 4825-4832.
- Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci. 2000; 20(2): 660-665.
- Van Es MA, Goedee HS, Westeneng HJ, Nijboer TCW, van den Berg LH. Is it accurate to classify ALS as a neuromuscular disorder? Expert Rev Neurother. 2020; 20(9): 895-906.
- Fischer LR, Culver DG, Tennant P et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004; 185(2): 232-240.
- Eisen A, Weber M. The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve. 2001; 24(4): 564-573.
- Tsitkanou S, Della Gatta PA, Russell AP. Skeletal muscle satellite cells, mitochondria, and micrornas: their involvement in the pathogenesis of ALS. Front Physiol. 2016; 7: 403.
- De Diego AMG, García AG. Altered exocytosis in chromaffin cells from mouse models of neurodegenerative diseases. Acta Physiol (Oxf). 2018; 224(2): e13090.
- Chida K, Sakamaki S, Takasu T. Alteration in autonomic function and cardiovascular regulation in amyotrophic lateral sclerosis. J Neurol. 1989; 236(3): 127-130.
- Baltadzhieva R, Gurevich T, Korczyn AD. Autonomic impairment in amyotrophic lateral sclerosis. Curr Opin Neurol. 2005; 18(5): 487-493.
- Kandinov B, Korczyn AD, Rabinowitz R, Nefussy B, Drory VE. Autonomic impairment in a transgenic mouse model of amyotrophic lateral sclerosis. Auton Neurosci. 2011; 159(1-2): 84-89.
- Ziegler MG, Brooks BR, Lake CR, Wood JH, Enna SJ. Norepinephrine and gamma-aminobutyric acid in amyotrophic lateral sclerosis. Neurology. 1980; 30(1): 98-101.
- Kandinov B, Grigoriadis NC, Touloumi O et al. Immunohistochemical analysis of sympathetic involvement in the SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013; 14(5-6): 424-433.
- Sachs C, Conradi S, Kaijser L. Autonomic function in amyotrophic lateral sclerosis: a study of cardiovascular responses. Acta Neurol Scand. 1985; 71(5): 373-378.
- Marin B, Desport JC, Kajeu P et al. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry. 2011; 82(6): 628-634.
- González de Aguilar JL, Gordon JW, Rene F et al. A mouse model of familial amyotrophic lateral sclerosis expressing a mutant superoxide dismutase 1 shows evidence of disordered transport in the vasopressin hypothalamo-neurohypophysial axis. Eur J Neurosci. 1999; 11(12): 4179-4187.
- De Diego AM, Lorrio S, Calvo-Gallardo E, García AG. Smaller quantal size and faster kinetics of single exocytotic events in chromaffin cells from the APP/PS1 mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2012; 428(4): 482-486.
- Mackenzie KD, Duffield MD, Peiris H et al. Huntingtin-associated protein 1 regulates exocytosis, vesicle docking, readily releasable pool size and fusion pore stability in mouse chromaffin cells. J Physiol. 2014; 592(7): 1505-1518.
- Calvo-Gallardo E, Pascual R de , Fernández-Morales JC et al. Depressed excitability and ion currents linked to slow exocytotic fusion pore in chromaffin cells of the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Am J Physiol Cell Physiol. 2015; 308(1): C1-19.
- Martínez-Ramírez C, Baraibar AM, Nanclares C et al. Altered excitability and exocytosis in chromaffin cells from the R6/1 mouse model of Huntington’s disease is linked to overexpression of mutated huntingtin. J Neurochem. 2018; 147(4): 454-476.
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391(2): 85-100.
- Wightman RM, Jankowski JA, Kennedy RT et al. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991; 88(23): 10754-10758.
- Hirano A, Donnenfeld H, Sasaki S, Nakano I. Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984; 43(5): 461-470.
- Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000; 529(Pt1): 57-68.
- Martin LJ. Mitochondriopathy in Parkinson disease and amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006; 65(12): 1103-1110.
- Montero M, Alonso MT, Carnicero E et al. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol. 2000; 2(2): 57- 61.
- Méndez-López I, Sancho-Bielsa FJ, Engel T, García AG, Padín JF. Progressive mitochondrial sod1(g93a) accumulation causes severe structural, metabolic and functional aberrations through opa1 down-regulation in a mouse model of amyotrophic lateral sclerosis. Int J Mol Sci. 2021; 22(15): 8194
- Ruiz-Ruiz C, Calzaferri F, García AG. P2X7 Receptor antagonism as a potential therapy in amyotrophic lateral sclerosis. Front Mol Neurosci. 2020; 13: 93.
- DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016; 139(Suppl 2): 136-153.
- Gandelman M, Levy M, Cassina P, Barbeito L, Beckman JS. P2X7 receptor-induced death of motor neurons by a peroxynitrite/FAS-dependent pathway. J Neurochem. 2013; 126(3): 382-388.
- Apolloni S, Parisi C, Pesaresi MG et al. The NADPH oxidase pathway is dysregulated by the P2X7 receptor in the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J Immunol. 2013; 190(10): 5187-5195.
- Fabbrizio P, Amadio S, Apolloni S, Volonte C. P2X7 receptor activation modulates autophagy in sod1-g93a mouse microglia. Front Cell Neurosci. 2017; 11: 249.
- Ruiz-Ruiz C, García-Magro N, Negredo P et al. Chronic administration of P2X7 receptor antagonist JNJ-47965567 delays disease onset and progression, and improves motor performance in ALS SOD1(G93A) female mice. Dis Model Mech. 2020; 13(10).
CONFLICT OF INTEREST STATEMENT
The authors of this article declare that they have no conflict of interest with respect to what is expressed in this work.
Autor para la correspondencia
Antonio G. García
Tlf.: +34 497 53 84 | E-Mail: agg@uam.es
Año 2022 · número 139 (01) · páginas 78 a 87
Enviado: 28.09.21
Revisado: 03.04.22
Aceptado: 12.04.22


